Posted on almost 8 years ago by Larry O'Leary
A study of healthy, middle-aged adults has found that poor sleep can raise levels of two proteins associated with Alzheimer's disease: amyloid beta and tau. Just 1 night of disrupted deep, slow wave sleep raised levels of amyloid beta, while a week of poor-quality sleep raised levels of tau.
The researchers - from Washington University School of Medicine in St. Louis, MO, Stanford University in Palo Alto, CA, and Radboud University Medical Centre in the Netherlands - describe their study in the journal Brain.
The team believes that the findings support the idea that chronic poor sleep in midlife could raise the risk of developing Alzheimer's disease later on.
Co-first author Yo-El Ju, an assistant professor of neurology at Washington University School of Medicine, says that she thinks it is unlikely that just 1 night, or even 1 week, of poor-quality sleep has much impact on Alzheimer's risk. The protein levels probably return to normal afterwards.
"The main concern," she says, "is people who have chronic sleep problems. I think that may lead to chronically elevated amyloid levels, which animal studies have shown lead to increased risk of amyloid plaques and Alzheimer's."
Abnormal protein structures
Alzheimer's disease is the most common cause of dementia, a progressive neurological disease that affects memory, thinking, decision-making, language, and speech, among other brain functions.
There are more than 5.5 million people living with Alzheimer's disease in the U.S., and this number is projected to rise to 16 million by 2050.
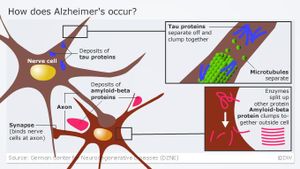
The brains of people with Alzheimer's disease feature two types of abnormal, insoluble protein structures: clumps called amyloid plaques that build up outside brain cells, and tangles of tau protein that build up inside brain cells.
The buildup of these two types of abnormal structure causes brain tissue to degenerate and die, and it also correlates with the progression of behavioral symptoms of the disease.
There is also mounting evidence to suggest that amyloid beta and tau, the two soluble proteins behind plaques and tangles, also "work together, independently of their accumulation into plaques and tangles, to drive healthy neurons into the diseased state."
The need to investigate sleep quality
Around a third of adults in the U.S. report that they usually get less sleep than the amount recommended.
Not getting enough sleep is a recognized threat to public health. It not only raises the risk of road crashes and mistakes at work that can lead to injury, but it is also tied to higher risk of a number of chronic diseases such as heart disease, diabetes, obesity, and depression.
In their study paper, Prof. Ju and colleagues highlight research in humans and mice that has linked sleep disruption and sleep deprivation to Alzheimer's disease, as well as to amyloid beta and tau.
For example, people with apnea - a sleep disorder that causes repeated cessation of airflow at night - have a higher risk of developing mild cognitive impairment (MCI) some 10 years earlier than people without apnea.
People with MCI show a slight but measurable decline in memory, thinking, and other mental skills and are more likely to develop Alzheimer's disease.
But the team notes that, despite the extent of the research, none has yet investigated which aspect of sleep might be responsible.
They make a case for investigating slow wave activity (SWA), a type of deep sleep that differs from rapid eye movement (REM) and that helps us to wake up feeling refreshed and rested. They describe SWA as a "strong candidate" for influencing levels of amyloid beta.
SWA sleep is thought to be a resting time, wherein the brain clears away molecular waste products that build up in brain cells during the day, when they are active.
Study designed to disrupt SWA sleep
Thus, for their study, the team recruited 22 healthy adults aged between 35 and 65, all of whom reported experiencing no sleep problems and who had no cognitive impairments. They asked them to wear wrist monitors for up to 2 weeks at a time. The wrist monitors measured how much time at night was spent asleep.
After they had worn the wrist monitors for 5 or more nights in a row, the participants individually spent the night in a room designed to observe and measure different aspects of sleep. They did this twice, with 28 days elapsing between the single-night visits. The difference between the two visits was that on one, the sleep was deliberately disrupted by the researchers, but on the other, it was not.
The comfortable, sound-proof room is climate-controlled and designed to create the conditions for one person to have a good night's sleep.
As they slept in the room, the participants wore earphones through which the researchers could deliver various sounds, and they also wore electrodes on their scalp to measure brain wave patterns. This allowed the team to time the disruption to periods of particular brain wave patterns.
The participants were split into two groups. For those in the first group, their first night in the sleep room was disturbed by the researchers, and then during the second night, around 4 weeks later, they were undisturbed. For the participants in the other group, the pattern was reversed: the first night was undisturbed while the second night was disturbed.
The researchers delivered the sleep disruption through the earphones as a series of beeps that gradually increased in volume. The noises were delivered when the participants' brain wave pattern showed that they were in deep, dreamless, non-REM, SWA sleep. The beeps continued until the SWA pattern subsided and the brain waves showed that they were in shallower sleep.
Disrupted SWA raised amyloid beta
The results showed that the morning after having their SWA disrupted, participants reported feeling unrefreshed and tired. This was the case even though they could rarely recall being woken in the night, and they had slept the same length of time that was normal for them.
Following a night in the sleep room, the participants also underwent a lumbar puncture to remove a sample of spinal fluid from which the researchers could measure levels of amyloid beta and tau proteins.
When they analyzed the results, the team found that levels of amyloid beta were 10 percent higher following a single night of disrupted SWA sleep compared with a night of undisturbed sleep. But there was no corresponding rise in tau.
However, the team found spikes in the tau levels of participants whose wrist monitors showed that they had experienced poor-quality sleep in the week leading up to the lumbar puncture.
Prof. Ju says that they were not surprised that tau levels did not spike after only 1 night of sleep disruption whereas this did cause amyloid levels to rise, since tau levels tend to change more slowly. "But we could see," she adds, "when the participants had several bad nights in a row at home, that their tau levels had risen."
"At this point, we can't say whether improving sleep will reduce your risk of developing Alzheimer's. All we can really say is that bad sleep increases levels of some proteins that are associated with Alzheimer's disease. But a good night's sleep is something you want to be striving for anyway."
Prof. Yo-El Ju
Source: Medical News Today