Posted on about 5 years ago by Laurentina Kennedy
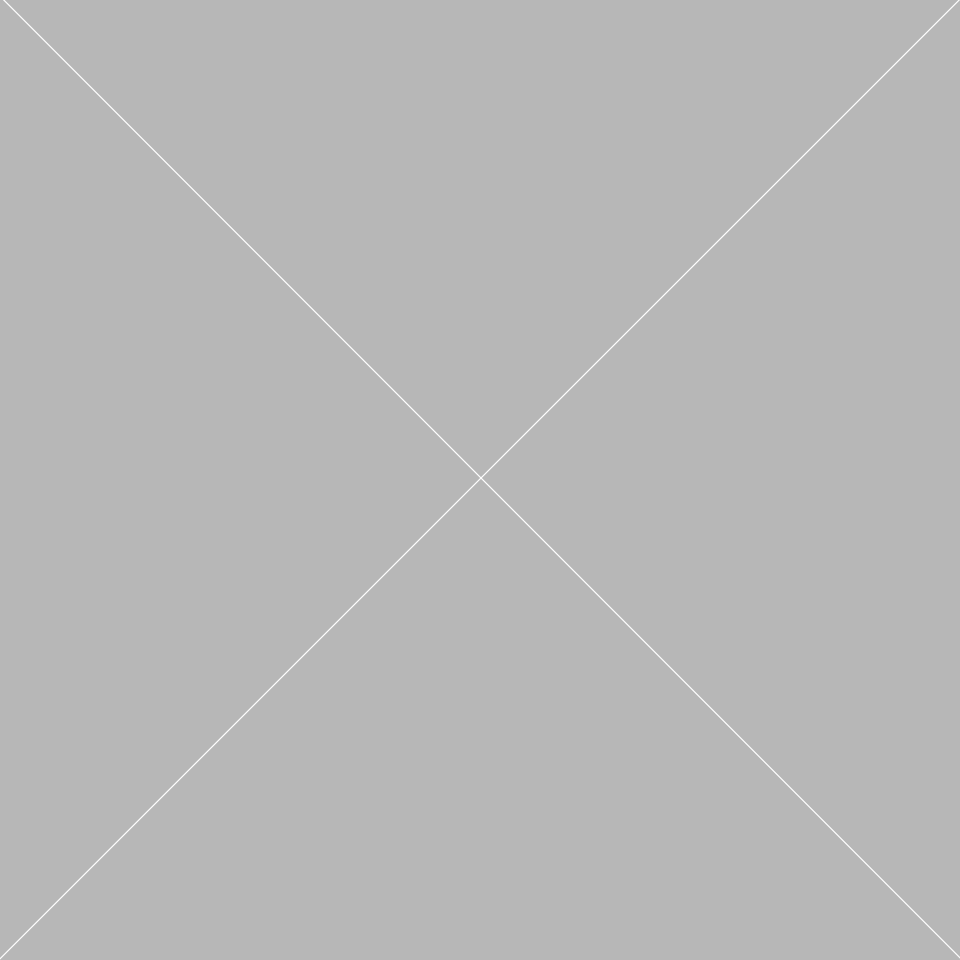
EU agrees deal for up to 160 million doses of Moderna Covid-19 vaccine
The European Union has reached a deal with US biotech firm Moderna for the supply of up to 160 million doses of its Covid-19 vaccine candidate, the President of the European Commission has said.
The deal will be formally approved by the EU executive tomorrow, Ursula von der Leyen said.
"According to the results of clinical trials, this vaccine could be highly effective against Covid-19," European Commission President Ursula von der Leyen said.
"Once the vaccine is indeed proven as safe and effective, every member state will receive it at the same time on a pro-rata basis," she said, in a brief statement.
Details of the contract, including any purchase options within the 160 million possible total number of doses, will be announced when it is signed.
Brussels has previously signed contracts for hundreds of millions of doses of possible future vaccines from Johnson & Johnson, Sanofi-GSK, AstraZeneca, Pfizer-BionNTech and CureVac.
Ms Von der Leyen has said that the European Medicines Agency may next month give approval for the most promising vaccines, that have already submitted data from clinical trials.
"Between us we are setting up one of the most comprehensive Covid-19 vaccine portfolios in the world. This provides Europeans access to the most promising future vaccines under development so far," she said.
"Of course, all vaccines from our portfolio will be evaluated very carefully by our European Medicines Agency, the EMA," she added.
"They will be only authorised and placed on the market if they are safe and if they are effective. Transparency here is crucial and of utmost importance."
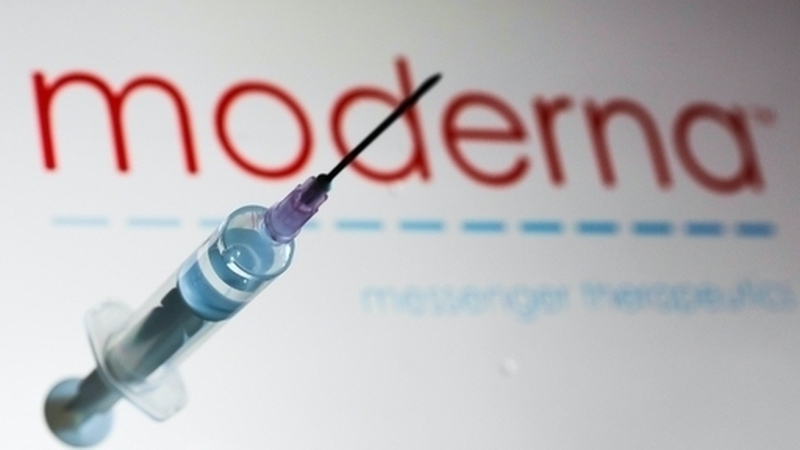
Meanwhile, Russia's Sputnik V coronavirus vaccine is 95% effective according to a second interim analysis of clinical trial data, its developers said.
The two-dose vaccine will be available on international markets for less than €8.40 per dose, they said, and will be free for Russian citizens.
It can be stored at between 2 and 8C (35.6-46.4F), they said, instead of the temperatures below freezing required for some other vaccines.
The calculations of its effectiveness were based on preliminary data obtained 42 days after the first dose, Russia's health ministry, the state-run Gamaleya research centre and the Russian Direct Investment Fund (RDIF) said in a statement.
The statement said that the vaccine had shown 91.4% effectiveness 28 days after the first dose.
The findings, which are yet to be peer-reviewed, is from new clinical trial data based on 39 confirmed cases and 18,794 patients.
Forty-two days later, after a second dose, data showed "an efficacy of the vaccine above 95%."
It did not note the number of coronavirus cases used to make the final calculation, however.
"The second analysis was conducted a week after volunteers got the second dose, meaning that their bodies have partially reacted to both doses," Gamaleya's director Alexander Gintsburg said in the statement.
He said the centre expects the efficacy rate to be "even higher" three weeks after the second dose.
The statement said that 22,000 volunteers had been vaccinated with the first dose and more than 19,000 with both doses.
Commenting on the findings, Ian Jones, professor of virology at the University of Reading, said: "The Sputnik V vaccine data looks impressive especially as the number of participants is high and the data was analysed only seven days after the second dose, too soon for the immune response to the boost to be fully in effect.
"On the face of it the data would appear to have the edge on the Oxford/AZ trial as the Oxford 90% protection figure was observed in only a subset of trial participants who received a lower first dose.
"However, a full comparison will only be possible when all the data is released."
Overseas trials of the vaccine are also taking place in the United Arab Emirates, Venezuela, Belarus and other countries.
Russia in August became the first country to register a coronavirus vaccine but did so ahead of the large-scale clinical trials that are still under way.
Last month, President Vladimir Putin announced that Russia had registered a second coronavirus vaccine, EpiVacCorona, as a global race heats up in producing an effective vaccine to combat the pandemic, which has now claimed the lives of nearly 1.4 million people.
Mr Putin last week said that Russia had manufacturing agreements in place with China and India and encouraged Brazil and South Africa to also mass produce Russian-made vaccines.
Pharma giants Pfizer and BioNTech announced that their virus vaccine is 95% effective, while US company Moderna said last week early results showed its candidate was 94.5% effective.